Microglial research is at the forefront of understanding the brain’s immune system and its pivotal role in neurodegenerative diseases like Alzheimer’s disease. These specialized cells, known as microglia, act as the guardians of the brain, constantly surveying for signs of inflammation and damage. Recent studies spearheaded by renowned neuroscientist Beth Stevens have revealed that while microglia are crucial for maintaining brain health, abnormal functioning can lead to detrimental effects, including the progression of conditions such as Alzheimer’s and Huntington’s disease. As we delve deeper into the mechanisms of microglial activity, it becomes increasingly clear that targeting these immune cells could pave the way for groundbreaking therapies and biomarkers. By shedding light on the complexities of microglia, researchers are not only enhancing our understanding of neurodegenerative diseases but are also providing hope for the estimated 7 million people in the U.S. living with Alzheimer’s.
In the realm of brain health, the study of glial cells has gained significant attention as researchers explore their functions in neurodegenerative conditions. These brain resident immune cells, often referred to as the brain’s custodians, play a vital role in monitoring neural environments, facilitating neuronal connections, and clearing cellular debris. Investigators like Beth Stevens have initiated transformative projects that examine how misbehaving microglia can contribute to the pathophysiology of Alzheimer’s and other neurodegenerative disorders. As the understanding of these immune mechanisms evolves, it holds the potential to inform novel therapeutic strategies that could alter the course of such debilitating diseases. By focusing on the subtle interactions of the brain’s cellular landscape, scientists are unveiling a new frontier in medical research with promising implications for patient outcomes.
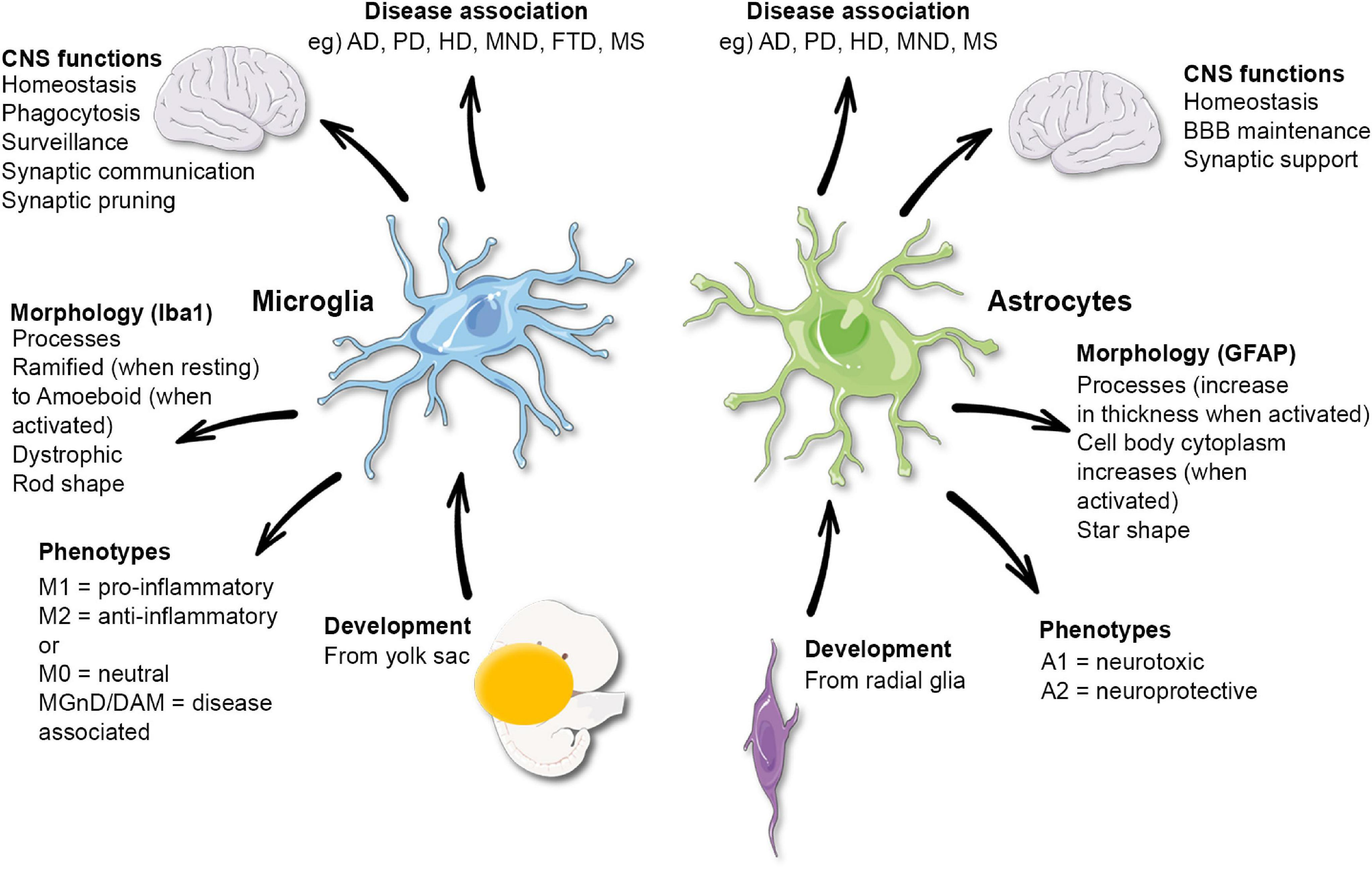
Understanding Microglial Cells in Neurodegenerative Diseases
Microglial cells have emerged as pivotal players in the fight against neurodegenerative diseases such as Alzheimer’s. These cells, which act as the brain’s immune system, are responsible for monitoring the health of neurons and responding to injury or disease. They perform critical functions, including the removal of dead or damaged neurons and the pruning of synapses, which are essential for maintaining proper neural connections. However, when microglia become overactive or dysfunctional, they can inadvertently contribute to neurodegenerative processes, leading to diseases like Alzheimer’s by improperly pruning synapses and exacerbating neuronal loss.
Research spearheaded by scientists like Beth Stevens has illuminated the dual role of microglial cells in both aiding brain health and potentially fostering disease. For instance, Stevens’ work has demonstrated that abnormal microglial function can accelerate the progression of conditions such as Alzheimer’s and Huntington’s disease. This revelation has significant implications for developing new therapeutic strategies that target microglial activity, aiming to restore their protective functions while mitigating their harmful effects. Understanding these mechanisms is crucial in the quest to develop effective treatments for millions of individuals affected by neurodegenerative diseases.
Beth Stevens: Transforming Alzheimer’s Research
Beth Stevens, an influential neuroscientist at Harvard Medical School, has transformed the landscape of Alzheimer’s research through her groundbreaking investigations into microglial cells. Her curiosity-driven approach has unlocked new insights into the immune system of the brain, revealing complex interactions between microglia and neurons during both development and disease. Her discoveries underscore that the health of the brain is intrinsically linked to the functioning of its immune components, challenging traditional views of neurodegeneration and opening new avenues for therapeutic intervention.
Recognized as a MacArthur ‘genius’ for her contributions to microglial research, Stevens emphasizes the broader implications of her studies beyond immediate clinical applications. By studying microglia in model organisms, her lab elicits fundamental biological questions that can’t be explored in human subjects directly. This basic science is vital for understanding the underpinnings of neurodegenerative diseases and ultimately paves the way for innovations in treatment. Stevens’ work exemplifies how investigation into the immune dynamics of the brain can lead to not only improved diagnostics but also novel therapeutic strategies to combat Alzheimer’s disease.
The Role of Microglia in Aging and Dementia
As the population ages, the prevalence of neurodegenerative diseases, particularly Alzheimer’s, continues to rise. Microglia play a crucial role in the aging brain, adapting their functions to respond to age-related changes. Research indicates that while microglia are essential for maintaining neural health, their activity can become dysregulated with age, leading to chronic inflammation and neuronal damage. Understanding how aging affects microglial function is paramount for uncovering the mechanisms underlying late-onset Alzheimer’s disease.
Recent studies suggest that interventions aimed at enhancing microglial function may hold promise for mitigating age-related cognitive decline. By promoting the health of these critical immune cells, scientists hope to prevent or slow the progression of dementia. This line of research not only highlights the potential of microglial-targeted therapies but also underscores the need for further investigation into the aging brain’s immune response. Through innovative studies, researchers are working to harness the protective capabilities of microglia and translate these findings into effective Alzheimer’s treatments.
Microglial Dysfunction and Alzheimer’s Pathology
The pathology of Alzheimer’s disease is closely linked with microglial dysfunction. Abnormal activation of these immune cells can lead to a cascade of inflammatory responses that contribute to neurodegeneration. Research has shown that when microglia are unable to properly clear amyloid-beta plaques—hallmarks of Alzheimer’s—they can exacerbate neuronal damage, further accelerating cognitive decline. Understanding the underlying mechanisms of microglial dysfunction is key to identifying potential biomarkers for early detection.
Contemporary research efforts are focused on developing therapies that can restore normal microglial function, potentially reversing some of the damage caused by Alzheimer’s. By targeting the inflammatory pathways associated with microglial activity, scientists aim to create treatments that not only reduce the burden of amyloid plaques but also promote overall neural health. These approaches highlight the imperative of integrating basic science with clinical research to effectively combat neurodegenerative diseases like Alzheimer’s.
The Future of Alzheimer’s Research: Insights from Microglial Studies
The future of Alzheimer’s research is being shaped significantly by insights gained from microglial studies. As researchers continue to uncover the complex roles that microglia play in brain health and disease, new therapeutic avenues are emerging. Innovations in our understanding of how these immune cells function during the progression of Alzheimer’s could lead to breakthroughs in treatments that not only slow the disease but also improve the quality of life for those affected.
Moreover, as awareness grows about the importance of brain immune responses, funding and resources are increasingly directed toward this vital area of research. Scientists are exploring novel ways to harness the protective roles of microglia while minimizing their detrimental effects. The integration of microglial research with ongoing studies into other aspects of neurodegenerative diseases is essential for devising comprehensive strategies to tackle Alzheimer’s and similar conditions effectively.
Exploring Biomarkers in Microglial Research
The exploration of biomarkers linked to microglial activity is a significant frontier in Alzheimer’s research. Discovering reliable biomarkers could enable early diagnosis of Alzheimer’s disease, allowing for timely intervention. Microglia have been shown to alter their expression profiles in response to neurodegeneration, making them prime candidates for biomarker development. By identifying specific microglial markers associated with Alzheimer’s progression, researchers hope to track disease progression more accurately and personalize treatment strategies.
Moreover, the characterization of these biomarkers can facilitate clinical trials aimed at testing new therapeutic agents targeting microglial dysfunction. As more is understood about the role of microglia in neuroinflammation and synaptic pruning, the potential to create diagnostic tools that integrate these findings into clinical practice becomes increasingly viable. This focus on biomarker research highlights the interdisciplinary nature of modern Alzheimer’s investigations, combining insights from immunology, neurology, and molecular biology to forge a path toward effective patient care.
The Impact of Federal Support on Microglial Research
Federal funding plays a pivotal role in advancing microglial research and, by extension, Alzheimer’s studies. As highlighted by Beth Stevens’ success, support from institutions like the NIH has been crucial in transforming basic scientific inquiries into impactful clinical outcomes. This financial backing allows researchers to explore complex issues surrounding microglial functions, diving deep into how they impact neurodegeneration and the progression of diseases like Alzheimer’s.
The collaboration between federal funding agencies and research institutions fosters an environment ripe for innovation, enabling investigators to delve into previously uncharted territories of neuroscience. With a firm financial foundation, scientists can pursue hypotheses that have the potential to yield transformative insights into the brain’s immune system and its relationship with Alzheimer’s disease. Continued investment in this area remains essential for translating scientific discoveries into viable treatments for those affected by neurodegenerative diseases.
Innovative Therapies Targeting Microglial Dysfunction
In recent years, innovative therapies targeting microglial dysfunction have gained attention as potential strategies for treating Alzheimer’s disease. By harnessing the natural ability of microglia to respond to neuronal injury and clear toxic substances, researchers are developing drugs that enhance these protective mechanisms. This therapeutic approach seeks not only to reduce neuroinflammation but also to promote neural repair, presenting a dual benefit in the management of Alzheimer’s pathology.
Studies have already started to explore the efficacy of such treatments, showcasing encouraging results in preclinical models. These therapies aim to restore the balance of microglial activity, ensuring that while they continue to protect the brain from injury, they do not initiate harmful inflammatory responses. As more clinical trials commence, the potential for translating these innovative approaches into real-world treatments could revolutionize the landscape of care for individuals with Alzheimer’s and other neurodegenerative diseases.
The Intersection of Basic Science and Clinical Application in Microglial Research
The relationship between basic science and clinical application is crucial in the realm of microglial research related to Alzheimer’s disease. Fundamental discoveries regarding the roles of microglia have provided a robust foundation for understanding how brain immune responses can influence neurodegenerative processes. For scientists like Beth Stevens, the initial curiosity-driven research drives a cycle of knowledge expansion that facilitates clinical breakthroughs. This interplay supports the development of new therapeutic strategies aimed at targeting the microglial responses in Alzheimer’s.
Moreover, as researchers recognize the relevance of microglial dysfunction to the pathophysiology of Alzheimer’s, the focus shifts towards translating this knowledge into actionable clinical applications. Enabling this translation requires collaboration across disciplines, integrating preclinical findings with patient-centered research. This interconnected approach not only enhances our understanding of Alzheimer’s disease but also positions microglial research at the forefront of innovative treatment development.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial components of the brain’s immune system, monitoring for signs of illness and injury, particularly in Alzheimer’s disease. Research led by Beth Stevens highlights how these cells assist in the clearance of damaged cells and prunes synapses, processes that can become dysfunctional and contribute to neurodegenerative diseases like Alzheimer’s.
How can abnormal microglial activity contribute to neurodegenerative diseases?
Abnormal microglial activity, such as excessive synaptic pruning, has been shown to contribute to neurodegenerative diseases, including Alzheimer’s and Huntington’s disease. This abnormal pruning can disrupt neural connections, leading to cognitive decline and other symptoms associated with these conditions.
What are the implications of Beth Stevens’ microglial research for treating neurodegenerative diseases?
Beth Stevens’ microglial research aims to uncover new biomarkers and therapeutic strategies for treating neurodegenerative diseases, especially Alzheimer’s. Her findings suggest that manipulating microglial functions could lead to novel approaches for detecting and potentially mitigating the impacts of these diseases.
What advancements have been made in understanding the brain immune system through microglial research?
Recent advancements in microglial research, particularly by Beth Stevens, have transformed our understanding of the brain’s immune system. These cells not only protect the brain by clearing debris but also sculpt neural circuits, indicating their dual role in both health and disease management, notably in Alzheimer’s.
Why is basic microglial research important for future Alzheimer’s disease therapies?
Basic microglial research is vital for the future of Alzheimer’s disease therapies because it lays the foundation for understanding disease mechanisms. As Beth Stevens emphasizes, curiosity-driven science can lead to significant discoveries that ultimately enhance treatment options for patients suffering from neurodegenerative diseases.
How do microglia influence synaptic pruning during brain development?
Microglia play a critical role in synaptic pruning during brain development by eliminating excess synapses to refine neural circuits. This process is essential for normal cognitive function and is being explored in the context of how disruptions in this function can lead to neurodegenerative diseases like Alzheimer’s.
What funding sources support microglial research into Alzheimer’s disease?
Much of the microglial research into Alzheimer’s disease, such as that conducted by Beth Stevens, has been supported by significant federal funding, including the National Institutes of Health (NIH). This support enables researchers to investigate critical pathways involved in neurodegenerative diseases.
| Key Points | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, maintaining health by monitoring for illness and clearing damaged cells. |
| Research Impact | Beth Stevens’ research has reshaped the understanding of microglial function and its connection to diseases like Alzheimer’s and Huntington’s. |
| Abnormal Pruning | Incorrect synaptic pruning by microglia can contribute to neurodegenerative diseases. |
| Research Funding | Stevens highlights the importance of NIH and federal funding in advancing her research. |
| Innovative Therapies | Findings have led to the development of new biomarkers and potential therapies for neurodegenerative diseases. |
Summary
Microglial research is vital to understanding brain health and combating neurodegenerative diseases. The work of Beth Stevens highlights how insight into microglial function not only enhances our knowledge of brain immunity but also paves the way for new treatments for conditions like Alzheimer’s disease. By supporting foundational research and the exploration of microglial activity, scientists are opening avenues for innovative therapies that could benefit millions suffering from these debilitating diseases.